Select pages - there are more than 4 available
Select pages - there are more than 4 available
Every Breath You Take.

Joseph Priestley.
1733 - 1804
Joseph Priestley was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted experiments in electricity and other areas of science. He was a close friend of, and worked in close association with Benjamin Franklin involving electricity experiments.
There isn't much evidence to support this claim.
Priestley is credited with his independent discovery of oxygen by the thermal decomposition of mercuric oxide, having isolated it in 1774. During his lifetime, Priestley's considerable scientific reputation rested on his invention of carbonated water, his writings on electricity, and his discovery of several "airs" (gases), the most famous being what Priestley dubbed "dephlogisticated air" (oxygen). Priestley's determination to defend phlogiston theory and to reject what would become the chemical revolution eventually left him isolated within the scientific community.
In August 1774 he allegedly isolated an "air" that appeared to be completely new, but he did not have an opportunity to pursue the matter because he was about to tour Europe with Shelburne. While in Paris, Priestley replicated the experiment for others, including French chemist Antoine Lavoisier. After returning to Britain in January 1775, he continued his experiments and discovered "vitriolic acid air" (sulphur dioxide, SO2).
In March he wrote to several people regarding the new "air" that he had discovered in August. One of these letters was read aloud to the Royal Society, and a paper outlining the discovery, titled "An Account of further Discoveries in Air", was published in the Society's journal Philosophical Transactions. Priestley called the new substance "dephlogisticated air", which he made in the famous experiment by focusing the sun's rays on a sample of mercuric oxide.
He first tested it on mice, who surprised him by surviving quite a while entrapped with the air, and then on himself, writing that it was "five or six times better than common air for the purpose of respiration, inflammation, and, I believe, every other use of common atmospherical air". He had discovered oxygen gas (O2).
Source: https://en.wikipedia.org/wiki/Joseph_Priestley

The Wikipedia entry for Shelburne has no reference to him going on a tour either by himself or with Priestley!
So this story cannot be taken seriously.
Electrostatic generator.
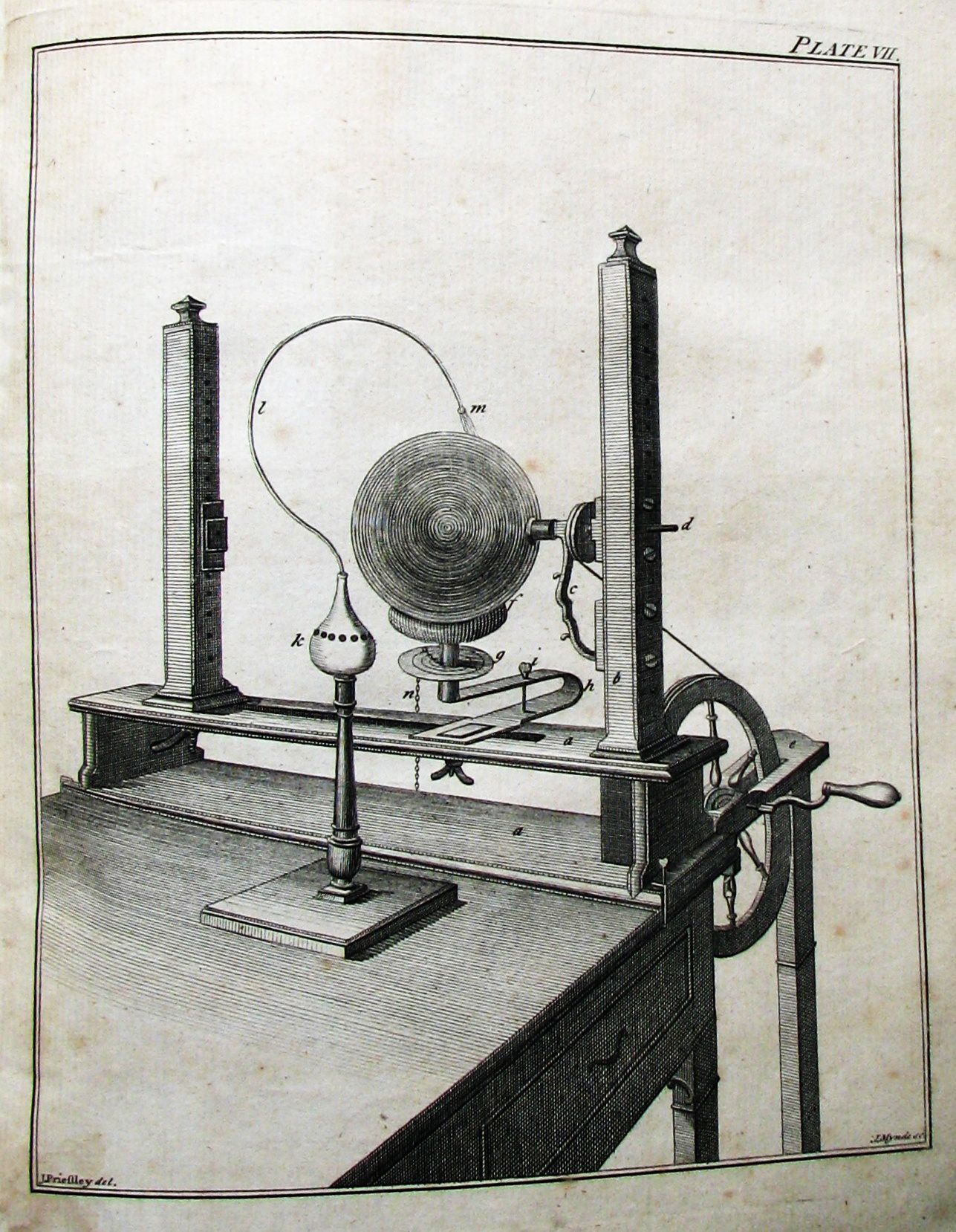
An engraving of the electrostatic machine that was allegedly invented and used by Priestley. The illustration appeared in the first edition of his publication: 'Familiar Introduction to the Study of Electricity' in 1768.
Like many designs of that period, it consists of a glass sphere that is rotated by a hand crank and the friciton generated by rubbing against a large felt pad, produces the static electricity.
Unlike a battery (an electrochemical cell), electrostatic generators cannot be used to provide a steady and reliable source of direct current voltage.
Luigi Galvani.
1735 - 1798
Luigi Galvani, (born Sept. 9, 1737, Bologna, Papal States—died Dec. 4, 1798, Bologna, Cisalpine Republic), Italian physician and physicist. His early research focused on comparative anatomy, including the structure of kidney tubules and the middle ear. His developing interest in electricity was inspired by the fact that dead frogs underwent convulsions when attached to an iron fence to dry. He experimented with muscular stimulation by electrical means, using an electrostatic machine and a Leyden jar, and from the early 1780s animal electricity remained his major field of investigation. His discoveries led to the invention of the voltaic pile, a kind of battery that makes possible a constant source of current electricity.
Source: https://www.britannica.com/summary/Luigi-Galvani
He was investigating the effects of distant atmospheric electricity (lightning) on prepared frog legs when he discovered that the legs convulsed not only when lightning struck, but also when he pressed the brass hooks attached to the frog's spinal cord to the iron railing they were suspended from.
Source: https://en.wikipedia.org/wiki/Luigi_Galvani
Galvani's apparatus used to strike frog's legs with lightning.

The lightning is supposed to strike the small rod of metal (marked B) and travel down the wire (marked A). The electrical force of the lightning then strikes the frog inside the jar (marked D). It also seems that lightning is supposed to strike the other piece of wire that is attached to the wall of the building, then strike the frog on the table.
The unpredictability of lightning strikes means that the likelihood of a strike hitting the small rod or piece of wire is almost zero!
If a strike did occur, then it is unlikely that it would be observed and the effects on the frogs would be catastrophic; so how can such an experiment be deemed valid?
A typical lightning strike has a voltage of around 300 million volts and a current of around 30,000 amps!
It's more back - projected lies and nobody has ever reproduced this experiment!

In his laboratory, Galvani later discovered that he could replicate this phenomena by touching metal electrodes of brass to the frog's spinal cord and to an iron plate. He concluded that this was proof of"animal electricity". the electric power of animated living things.
Pieter van Musschenbroek.
1692 - 1761
Pieter van Musschenbroek, a Dutch experimental scientist, was born Mar. 14, 1692. Musschenbroek’s father was an instrument maker, specializing in double-acting vacuum pumps, a family business that Pieter’s older brother continued. Not surprisingly, Pieter grew up with an interest in instrumental science, but he took the academic route, becoming a professor, first in Duisburg, then Utrecht, and then finally, in 1739, in Leiden. He was a prolific author of books on experimental science, many of which we have in our collections, in multiple editions. On Jan. 20, 1746, Musschenbroek wrote an excited letter to a French colleague, Réne-Antoine Ferchault de Réaumur. Musschenbroek began his letter (in Latin): “I would like to tell you about a new but terrible experiment, which I advise you never to try yourself, nor would I, who have experienced it and survived by the grace of God, do it again for all the kingdom of France.”
Source: https://www.lindahall.org/about/news/scientist-of-the-day/pieter-van-musschenbroek
Why would he write in Latin when Dutch was the language used by Dutch people of that time period and his colleague spoke French?
Source: https://en.wikipedia.org/wiki/History_of_the_Dutch_language
The monks who compiled the back - projected false history, spoke and wrote in Latin!
A typical Leyden Jar used to store electrical charge.

Musschenbroek had found a way to store electricity in a bottle. He discovered that if you half-fill a glass bottle with water (it must be German glass, he claimed!); insert a metal rod, with a knob on the end, into the jar; and hold the knob to a prime conductor connected to an electrostatic generator, you can fill the water with invisible electric fluid. You couldn't see it, but it was definitely there, and in prodigious quantity, because if you held the jar in your left hand, and touched the knob with your right, the shock would knock you off your feet. Musschenbroek had made what we would call an electrical capacitor. His contemporaries called it a Leyden jar.
It turns out that Musschenbroek did not invent the Leyden jar; he was preceded in his work by Jürgen von Kleist in far-off Pomerania. Nor did Musschenbroek discover the key to charging and discharging a Leyden jar – hold it in your hand; that was painfully discovered by a lawyer colleague of Musschenbroek. But Musschenbroek wrote the letter to Réaumur, who passed it on to the abbé Nollet, who read it to the Paris Academy of Sciences on Apr. 20, 1746, and successfully duplicated the experiment (he found that Parisian glass works just as well as German). It is said that Nollet coined the term “Leyden jar,” and perhaps he did, but I could not find the term in his published paper, although he makes copious use of the phrase “experience du Leyde”.

The picture above shows the beginning of the translation of Musschenbroek’s letter, You can perhaps make out the phrase at the top: “experience nouvelles, mais terrible” (for some reason the last half of Musschenbroek’s first sentence is elided – perhaps Nollet did not want to scare away future experimenters).
Or, perhaps it is a total fabrication!
Source: https://www.lindahall.org/about/news/scientist-of-the-day/pieter-van-musschenbroek
Alessandro Giuseppe Antonio Anastasio Volta .
1745 - 1827
Alessandro Giuseppe Antonio Anastasio Volta was an Italian physicist and chemist who allegedly was a pioneer of electricity and who is credited as the inventor of the electric battery and the discoverer of methane. He invented the voltaic pile in 1799, and reported the results of his experiments in 1800 in a two-part letter to the president of the Royal Society. With this invention Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments, which eventually led to the development of the field of electrochemistry.
Volta also drew admiration from Napoleon Bonaparte for his invention, and was invited to the Institute of France to demonstrate his invention to the members of the institute. Volta enjoyed a certain amount of closeness with the emperor throughout his life and he was conferred numerous honours by him.[1] Volta held the chair of experimental physics at the University of Pavia for nearly 40 years.
Source: https://en.wikipedia.org/wiki/Alessandro_Volta
Structure of a Galvanic Cell.

Volta's invention was built on Luigi Galvani's 1780s discovery of how a circuit of two metals and a frog's leg can cause the frog's leg to respond. Volta demonstrated in 1794 that when two metals and brine-soaked cloth or cardboard are arranged in a circuit they produce an electric current. In 1800, Volta stacked several pairs of alternating copper (or silver) and zinc discs (electrodes) separated by cloth or cardboard soaked in brine to increase the total electromotive force. When the top and bottom contacts were connected by a wire, an electric current flowed through the voltaic pile and the connecting wire.
Volta thought that the pile was a perpetual motion machine and he wrote:
"This endless circulation of the electric fluid (this perpetual motion) may appear paradoxical and even inexplicable, but it is no less true and real."
He never realised that the zinc discs 'oxidised' rapidly which resulted in the voltage dropping equally rapidly.
Once the electrolyte starts to dry out, the voltage falls rapidly.
Click on the images below to view them full size.
Because Volta believed that the electromotive force occurred at the contact between the two metals, Volta's piles had a different design than the modern design illustrated on this page. His piles had one extra disc of copper at the top, in contact with the zinc, and one extra disc of zinc at the bottom, in contact with the copper.
Adriaan Paets van Troostwijk.
1752 - 1837
Adriaan Paets van Troostwijk (4 March 1752 – 3 April 1837) was a Dutch businessman amateur chemist. He conducted experiments and theorized on the nature of substances, conducted some of the earliest experiments on the electrolysis of water in collaboration with physician Johan Rudolph Deiman (1743–1808).
Troostwijk was born in Utrecht to cloth-merchant Wouter van Troostwijk and Johanna Dolphina Paets. He married Marie Cornelia Loten in 1770 and joined the business of his father-in-law in Amsterdam until 1816 and lived in Niewersluis subsequently. Here he became a member of the Felix Meritis, an Amsterdam organization of polymaths founded in 1777. Along with his physician friend Jan Deiman, he conducted experiments and wrote 35 papers between 1778 and 1818.
The director of the Haarlem Teylers museum Martinus van Marum also collaborated with Paets van Troostwijk. Using an electrostatic generator, he was able to split water with gold as an electrode (acting as a catalyst as examined in studies in the 21st century) and was able to identify the components oxygen and hydrogen.
Source: https://en.wikipedia.org/wiki/Adriaan_Paets_van_Troostwijk
An electrostatic generator.

Using an electrostatic generator, he was allegedly able to split water into hydrogen and oxygen using gold electrodes.
The problem is that such a system will not produce a steady D.C. flow; only an intermittent, high voltage burst of electricity which is typically 50,000 volts!

Electrostatic generators are not suitable for such experiments as they do not produce a steady low voltage.
The paid authors who compiled the back - projected false history didn't care about or understand technical details!
William Nicholson.
1753 - 1815
William Nicholson was an English writer, translator, publisher, scientist, inventor, patent agent and civil engineer. He launched the first monthly scientific journal in Britain, Journal of Natural Philosophy, Chemistry, and the Arts, in 1797, and remained its editor until 1814. In 1800,
In 1799 he established a school in London's Soho Square, where he taught natural philosophy and chemistry, with the aid of a grant of £1,500 from Thomas Pitt.
In May 1800 he with Anthony Carlisle discovered electrolysis, the decomposition of water into hydrogen and oxygen by voltaic current. The two were then appointed to a chemical investigation committee of the new Royal Institution. But his own interests shortly turned elsewhere. In 1809 he became a first class corresponding member, living abroad, of the Royal Institute of the Netherlands.
Source: https://en.wikipedia.org/wiki/William_Nicholson_%28chemist%29
Anthony Carlisle.
1768 - 1840
Anthony Carlisle was born in Stillington, County Durham, the third son of Thomas Carlisle and his first wife, and the half-brother of Nicholas Carlisle. He was apprenticed to medical practitioners in York and Durham, including his uncle Anthony Hubback and William Green. He later studied in London under John Hunter. In 1793 he was appointed Surgeon at Westminster Hospital in 1793, remaining there for 47 years. He also studied art at the Royal Academy.
In 1800, he and William Nicholson discovered electrolysis by passing a voltaic current through water, decomposing it into its constituent elements of hydrogen and oxygen.
He was elected a Fellow of the Royal Society in 1804. He was Professor of Anatomy of the Society from 1808 to 1824.
In 1815 he became a member of the council of the Royal College of Surgeons,[1] and served as president of the College in 1828 and 1837. He twice delivered their Hunterian oration, causing consternation at his second oration in 1826 by using the occasion to talk about oysters, earning the epithet of Sir Anthony Oyster. He also delivered their Croonian Lecture in 1804, 1805 and 1807.
He was Surgeon Extraordinary (1820–1830) to King George IV, by whom he was knighted on 24 July 1821.
Source: https://en.wikipedia.org/wiki/Anthony_Carlisle
I smell a rat!
The rise of physical chemistry in the 19th century has at its root two closely connected events which took place in the final year of the 18th century. In 1800, Alessandro Volta in Lombardy invented an early form of battery, known as the Voltaic pile, which Messrs. Carlisle and Nicholson in England promptly employed to discover electrolysis.
Carlisle and Nicholson’s discovery that electricity can decompose water into hydrogen and oxygen caused as big a stir as any scientific discovery ever made. It demonstrated the existence of a relationship between electricity and the chemical elements, to which Michael Faraday would give quantitative expression in his two laws of electrolysis in 1834. Faraday also introduced the term ‘ion’, a little word for a big idea that Arrhenius, Ostwald and van ‘t Hoff would later use to create the foundations of modern physical chemistry in the 1880s.
The president of the Royal Society, Sir Joseph Banks, lived in a house at No.32 Soho Square. Here he entertained all the leading members of the scientific establishment, and it was here in April 1800 that he yielded to temptation and disclosed the contents of Signor Volta’s confidential letter to certain chosen acquaintances. Among them was another resident of Soho Square, the fashionable surgeon Anthony Carlisle, who had just moved in at No.12.
Volta’s announcement of his invention made an instant impression on Carlisle, who immediately arranged for his friend the chemist William Nicholson to look over the letter with him, after which Carlisle set about constructing the apparatus according to the instructions in Volta’s letter.
Nicholson records in his paper that by 30th April 1800, Carlisle had completed the construction of a pile “consisting of 17 half crowns, with a like number of pieces of zinc, and of pasteboard, soaked in salt water”. Using coinage for the silver discs was smart thinking by Carlisle – with a diameter of 1.3 inches (3.3 cm), the half crown was an ideal size for the purpose, and was made of solid silver.
From Nicholson’s account, it seems likely that Carlisle obtained a pound (approx. ½ kilo) of zinc from a metal dealer called John Tappenden who traded from premises just opposite the church of Saint Vedast Foster Lane, off Cheapside in the City of London. A pound of zinc was enough to make 20 discs of the diameter of a half crown.
On May 1st, 1800, Carlisle and Nicholson set up their pile – most likely in Carlisle’s house at 12 Soho Square – and began by forming a circuit with a steel wire and passing a current through it. To assist contact with the wire, a drop of river water was placed on the uppermost disc. As soon as this was done, Nicholson records
“Mr. Carlisle observed a disengagement of gas round the touching wire. This gas, though very minute in quantity, evidently seemed to me to have the smell afforded by hydrogen”
It is amazing that Nicholson was able to identify hydrogen from such a minute sample. But even more amazing was the thought that occurred to him next
“This [release of hydrogen gas], with some other facts, led me to propose to break the circuit by the substitution of a tube of water between two wires.”
Nicholson does not say what those other facts are, but he does record that on the first appearance of hydrogen gas, both he and Carlisle suspected that the gas stemmed from the decomposition of water by the electric current. Following that wonderfully intuitive piece of reasoning, Nicholson’s suggestion can be seen as a natural next step in their investigation.
Encyclopedia Britannica online.
hydrogen (H), a colourless, odourless, tasteless, flammable gaseous substance that is the simplest member of the family of chemical elements.
https://www.britannica.com/science/hydrogen
Nicholson could NOT have smelled hydrogen gas as it is odourless!
How did he know in advance what the gas was supposed to smell like?
Source: https://carnotcycle.wordpress.com/2017/02/01/carlisle-nicholson-and-the-discovery-of-electrolysis/
Chemical Flatulence!

According to mainstream scientific theory, it is possible to produce water by combining hydrogen and oxygen.
Somewhat surprisingly, it isn't just a case of mixing the two gases in a glass vessel, you have to explode the hydrogen gas by igniting it in air!

Images from the video.
Click on each one to read the comments.
In the video, Brian is seen adding concentrated hydrochloric acid to pieces of zinc metal to produce a very white coloured gas (hydrogen).
He makes bubbles of the gas by passing it through a soapy solution and then ignites the gas bubbles.
The flame produced is very orange in colour; so it can't be pure hydrogen.
He then fills a large glass vessel with the gas, but what is not shown is that he is using a commercial cylinder of (allegedly) hydrogen and not the gas he produced previously.
The gas is ignited using an electrical spark and the resultant explosion produces a small amount of condensation on the inside of the glass vessel.
It is important to note that when the gas is exploded in the air, it is said to produce water vapor. Water vapor is not liquid water; it is a gas.
KEY POINTS:
"When hydrogen and oxygen react during combustion, water vapor is produced."
https://en.wikipedia.org/wiki/Heat_of_combustion
"Water vapor, water vapour or aqueous vapor is the gaseous phase of water."
https://en.wikipedia.org/wiki/Water_vapor
The claim that liquid water is produced when hydrogen and oxygen are combined is a lie!
The video is a careful mix of different 'takes' and is designed to support the lie.
Quote from the video. Brian Cox - re Cavendish's experiment.
"Now Cavendish didn't really have any idea of what happened in these chemical reactions.
Indeed, his whole theoretical framework was nonsense to modern eyes. It was based on Alchemy.
He thought things burned because they contained a substance called phlogiston.
But even thought that is complete nonsense, because he was a great experimental scientist, his measurements were correct.
So, he managed to measure that water is made of two parts of hydrogen to one part of oxygen -H2O; even though he didn't believe that water was made of anything at all.
So, that ability to get your theoretical picture, your ideas about the way that nature works completely wrong and yet make honest and precise measurements that stand the test of time and are correct
is the mark of a great experimental scientist."
How can any sane person accept this drivel?
Exposing the lie.
The following illustrations are based on actual experiments. Anyone can reproduce the experiments as they use readily available materials and second hand plastic or glass bottles and containers can be used.
Thin cardboard can be used instead of a wooden splint.
The aluminium rods can be bought from a hardware or DIY store.
The aluminium foil is standard kitchen foil.
A small 9V D.C. or 12V D.C. power supply can be used or a battery.
The connecting wire can be bought from a hobby shop, DIY store or car parts shop.
De-ionised or distilled water can be bought from a car parts shop, DIY store or supermarket.








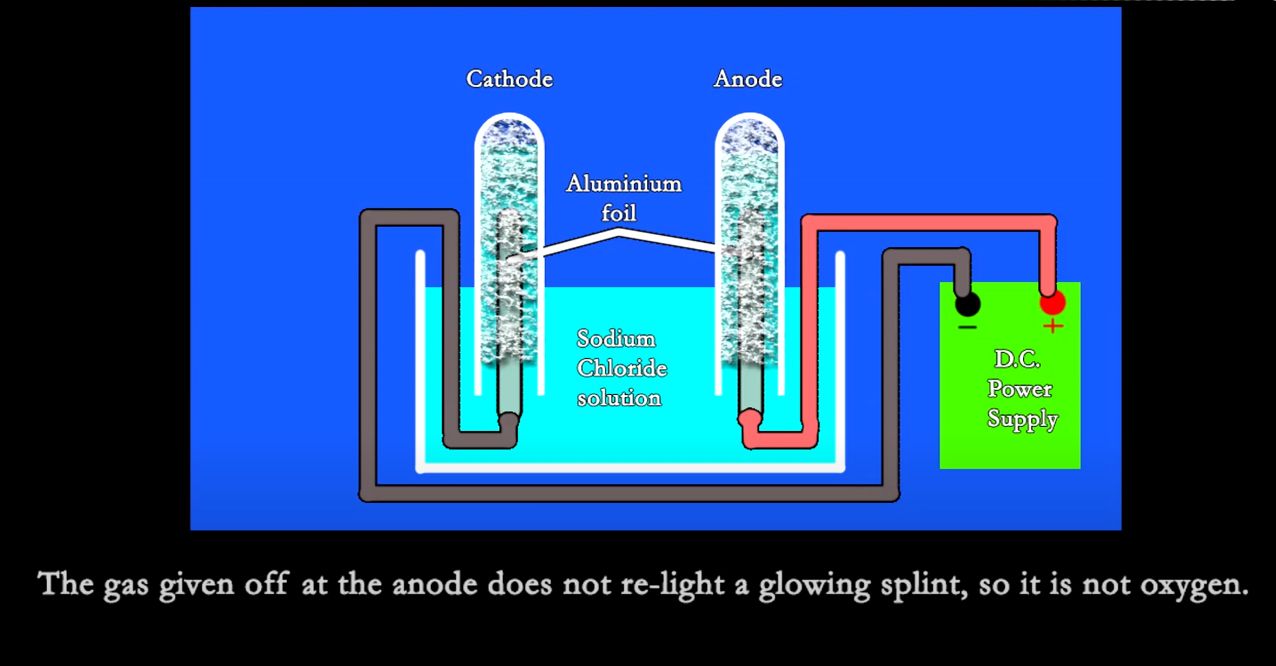

The hydrogen gas given off, is due to the decomposition of the aluminium used for the electrodes. The fact that chlorine gas is given off is due to the decomposition of the sodium chloride that was added to the distilled water.
Distilled or de-ionised water does not conduct electricity!
Water is NOT composed of hydrogen and oxygen, it is a pure element and NOT a compound.
It cannot be split into Hydrogen and Oxygen!

Select pages - there are more than 4 available
© Copyright 2022. All rights reserved.
We need your consent to load the translations
We use a third-party service to translate the website content that may collect data about your activity. Please review the details in the privacy policy and accept the service to view the translations.






















